Lessico
Amido

Amido di patata
Amido, in latino amylum/amolum, deriva dal greco ámylon ‘(farina) non macinata’, composto di a- privativa e mýlë ‘mola’. Si tratta di un composto chimico macromolecolare appartenente al gruppo dei polisaccaridi, costituito dall'unione di un gran numero di molecole di glucosio, C6H12O6, con l'eliminazione di una molecola di acqua per ogni molecola di glucosio. Di conseguenza l'amido viene rappresentato con la formula (C6H10O5)n. Il numero n, e cioè il numero dei residui di glucosio tra di loro uniti a formare le macromolecole degli amidi, è compreso, per un determinato tipo di amido, in un intervallo relativamente ristretto. Varia tuttavia da un amido all'altro, p. es. dall'amido di grano a quello di patate o a quello di riso, con un valore medio di poche centinaia.
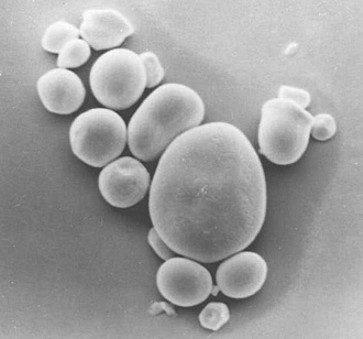
Amiloplastidi di cellule di patata al microscopio ottico
L'amido è il principale materiale di riserva delle piante, nelle cui cellule viene immagazzinato a livello di particolari formazioni citoplasmatiche (plastidi). Manca solo in alcune piante superiori (Composite), nei Funghi e nei Batteri, nelle Euglenofite, nelle Rodofite e nelle Cianofite.
L'amido si accumula soprattutto nelle radici, nei semi, nei tuberi sotto forma di granuli aventi dimensioni e aspetto molto variabili. All'osservazione microscopica i granuli presentano talora tipiche striature concentriche che circondano una zona detta ilo. Le caratteristiche morfologiche dei granuli di amido e la loro birifrangenza alla luce polarizzata costituiscono utili elementi per il riconoscimento di varie piante e hanno particolare interesse in campo chimico-bromatologico (branca della chimica che si occupa dell'analisi dei prodotti alimentari – dal greco brøma = cibo) per la determinazione del valore e della genuinità delle farine di cereali.
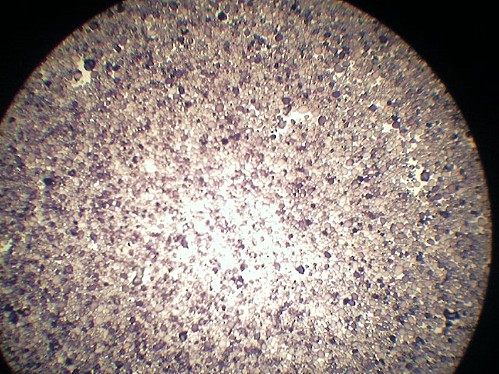
Foto
al microscopio di una patata.
Sono visibili gli agglomerati di amido dal colore scuro.
L'amido è una delle strutture più complesse tra i carboidrati che si formano nelle piante dalla fotosintesi clorofilliana. Insolubile in acqua, l'amido forma nell'acqua calda un liquido vischioso che, raffreddato, gelifica (salda d'amido). Per idrolisi parziale e per effetto della b-amilasi l'amido si trasforma in destrine. L'idrolisi totale, attuabile con acidi e con basi diluite oppure per via enzimatica (amilo-1,6-glucosidasi), porta alla formazione quantitativa di D-glucosio e di piccole quantità di acido fosforico, di ioni calcio, magnesio, sodio e potassio.
Il significato di tali elementi minerali reperibili negli idrolizzati d'amido non è noto. Si ritiene tuttavia che essi abbiano grande importanza nella determinazione dei processi di sintesi e di utilizzazione dell'amido nei vegetali. L'amido è in realtà costituito da due differenti complessi molecolari, l'amilosio e l'amilopectina, ambedue formati da residui di D-glucosio uniti mediante legami alfa-glucosidici.
L'amilosio, contenuto nell'amido in percentuale molto variabile, è formato da catene lineari di ca. 300 unità di glucosio legate tra loro in posizione (1->6). L'amilosio si colora intensamente in azzurro con iodio e non forma la salda.
L'amilopectina ha peso molecolare più elevato dell'amilosio, a differenza del quale forma la salda d'amido. Essa ha struttura ramificata e tridimensionale, in quanto nella sua molecola i legami tra le unità glucidiche avvengono non solo in posizione (1->6) ma anche in posizione (1->4). La beta-amilasi attacca l'amilosio scindendolo in maltosio. Al contrario, l'amilopectina subisce solo parzialmente la scissione amilasica.
Su scala industriale l'amido, utilizzato in applicazioni svariate, si estrae da materiali vegetali che ne sono particolarmente ricchi come le patate, il mais, il riso, ecc., disperdendoli in grande quantità d'acqua e separando l'amido per decantazione. L'amido rappresenta tra l'altro il prodotto di partenza per la preparazione industriale del glucosio, che da esso si ottiene per azione degli acidi minerali diluiti a caldo o di preparati enzimatici grezzi, come p. es. il malto, ricchi di enzimi amilolitici. L'amido trova impiego anche nell'industria tessile, in cosmetica, nella preparazione di colle e in campo farmaceutico. In medicina viene talora usato come antidoto esterno negli avvelenamenti da iodio.
Starch (CAS# 9005-25-8, chemical formula (C6H10O5)n) is a mixture of amylose and amylopectin (usually in 20:80 or 30:70 ratios). These are both complex carbohydrate polymers of glucose (chemical formula of glucose C6H12O6), making starch a glucose polymer as well, as seen by the chemical formula for starch, regardless of the ratio of amylose:amylopectin.
The word is derived from Middle English sterchen, meaning to stiffen, which is appropriate since it can be used as a thickening agent when dissolved in water and heated.
In terms of human nutrition, starch is by far the most consumed polysaccharide in the human diet. It constitutes more than half of the carbohydrates even in many affluent diets, and much more in poorer diets. Traditional staple foods such as cereals, roots and tubers are the main source of dietary starch.
Starch (in particular cornstarch) is used in cooking for thickening foods such as sauces. In industry, it is used in the manufacturing of adhesives, paper, textiles and as a mold in the manufacture of sweets such as wine gums and jelly beans. It is a white powder, and depending on the source, may be tasteless and odorless.
Starch is often found in the fruit, seeds, rhizomes or tubers of plants and is the major source of energy in these food items. The major resources for starch production and consumption worldwide are rice, wheat, corn, and potatoes. Cooked foods containing starches include boiled rice, various forms of bread and noodles (including pasta).
As an additive for food processing, arrowroot and tapioca are commonly used as well. Commonly used starches around the world are: arracacha, buckwheat, banana, barley, cassava, kudzu, oca, sago, sorghum, regular household potatoes, sweet potato, taro and yams. Edible beans, such as favas, lentils and peas, are also rich in starch.
When a starch is pre-cooked, it can then be used to thicken cold foods. This is referred to as a pregelatinized starch. Otherwise starch requires heat to thicken, or "gelatinize." The actual temperature depends on the type of starch.
A modified food starch undergoes one or more chemical modifications, which allow it to function properly under high heat and/or shear frequently encountered during food processing. Food starches are typically used as thickeners and stabilizers in foods such as puddings, custards, soups, sauces, gravies, pie fillings, and salad dressings, but have many other uses.
Resistant starch is starch that escapes digestion in the small intestine of healthy individuals.
Plants use starch as a way to store excess glucose, and thus also use starch as food during mitochondrial oxidative phosphorylation.

Starch adhesive
Papermaking is the largest non-food application for starches globally, consuming millions of metric tons annually. In a typical sheet of copy paper for instance, the starch content may be as high as 8%. Both chemically modified and unmodified starches are used in papermaking. In the wet part of the papermaking process, generally called the “wet-end”, starches are chemically modified to contain a cationic or positive charge bound to the starch polymer, and are utilized to associate with the anionic or negatively charged paper fibers and inorganic fillers. Starch also helps get out cleaning stains from dirty washing.
These cationic starches impart the necessary strength properties for the paper web to be formed in the papermaking process (wet strength), and to provide strength to the final paper sheet (dry strength). In the dry end of the papermaking process the paper web is rewetted with a solution of starch paste that has been chemically, or enzymatically depolymerized. The starch paste solutions are applied to the paper web by means of various mechanical presses (size press). The dry end starches impart additional strength to the paper web and additionally provide water hold out or “size” for superior printing properties.
Corrugating glues are the next largest consumer of non-food starches globally. These glues are used in the production of corrugated fiberboard (sometimes called corrugated cardboard), and generally contain a mixture of chemically modified and unmodified starches that have been partially gelatinized to form an opaque paste. This paste is applied to the flute tips of the interior fluted paper to glue the fluted paper to the outside paper in the construction of cardboard boxes. This is then dried under high heat, which provides the box board strength and rigidity.
Another large non-food starch application is in the construction industry where starch is used in the gypsum wall board manufacturing process. Chemically modified or unmodified starches are added to the stucco containing primarily gypsum. Top and bottom heavyweight sheets of paper are applied to the formulation and the process is allowed to heat and cure to form the eventual rigid wall board. The starches act as a glue for the cured gypsum rock with the paper covering and also provide rigidity to the board.
Clothing starch or laundry starch is a liquid that is prepared by mixing a vegetable starch in water (earlier preparations also had to be boiled), and is used in the laundering of clothes. Starch was widely used in Europe in the 16th and 17th centuries to stiffen the wide collars and ruffs of fine linen which surrounded the necks of the well-to-do. During the 19th century and early 20th century, it was stylish to stiffen the collars and sleeves of men's shirts and the ruffles of girls' petticoats by applying starch to them as the clean clothes were being ironed. Aside from the smooth, crisp edges it gave to clothing, it served practical purposes as well. Dirt and sweat from a person's neck and wrists would stick to the starch rather than fibers of the clothing, and would easily wash away along with the starch. After each laundering, the starch would be reapplied. Today the product is sold in aerosol cans for home use. Starch is also used to make some packing peanuts, and some dropped ceiling tiles.
Printing industry - in the printing industry food grade starch is used in the manufacture of anti-set-off spray powder used to separate printed sheets of paper to avoid wet ink being set off. Starch is also used in the manufacture of glues[3] for book-binding.
Hydrogen production - Starch can be used to produce Hydrogen.
Oil exploration - starch is used as to adjust the viscosity of drilling fluid which is used to lubricate the drill head in (mineral) oil extraction.
Gummed sweets such as jelly beans and wine gums are not manufactured using a mold in the conventional sense. A tray is filled with starch and leveled. A positive mold is then pressed into the starch leaving an impression of 1000 or so jelly beans. The mix is then poured into the impressions and then put into a stove to set. This method greatly reduces the number of molds that must be manufactured.
Starch can be modified by addition of some chemical forms to be a hard glue for paper work , some of those forms are Borax , Soda Ash , which mixed with the starch solution at 50-70 °C to gain a very good adhesive, Sodium Silicate can be added to reinforce this formula.
Iodine solution is used to test for Starch. A bluish-black color indicates the presence of iodine in the starch solution. It is thought that the iodine fits inside the coils of amylose. A 0.3% w/w solution is the standard concentration for a dilute starch indicator solution. It is made by adding 4 grams of soluble starch to 1 litre of heated water; the solution is cooled before use (starch-iodine complex becomes unstable at temperatures above 35 °C). This complex is often used in redox titrations: in presence of an oxidizing agent the solution turns blue, in the presence of reducing agent, the blue color disappears because triiodide (I3-) ions break up into three iodide ions, disassembling the complex.
Under the microscope, starch grains show a distinctive Maltese cross effect (also known as 'extinction cross' and birefringence) under polarized light.
Starch can be hydrolyzed into simpler carbohydrates by acids, various enzymes, or a combination of the two. The extent of conversion is typically quantified by dextrose equivalent (DE), which is roughly the fraction of the glycoside bonds in starch that have been broken. Food products made in this way include:
Maltodextrin, a lightly hydrolyzed (DE 10–20) starch product used as a bland-tasting filler and thickener.
Various corn syrups (DE 30–70), viscous solutions used as sweeteners and thickeners in many kinds of processed foods.
Dextrose (DE 100), commercial glucose, prepared by the complete hydrolysis of starch.
High fructose syrup, made by treating dextrose solutions to the enzyme glucose isomerase, until a substantial fraction of the glucose has been converted to fructose. In the United States, high fructose corn syrup is the principal sweetener used in sweetened beverages because fructose tastes sweeter than glucose, and less sweetener may be used.